New Protease Inhibitor Gains FDA Approval
On June 22, 2005, the US Food and Drug Administration (FDA) granted accelerated approval of APTIVUS (tipranavir), a protease inhibitor. APTIVUS, co-administered with 200 mg of ritonavir, is indicated for use as part of combination antiretroviral treatment of HIV-1 infected adult patients with evidence of viral replication, who are highly treatment-experienced or have HIV-1 strains resistant to multiple protease inhibitors.
The FDA reviewed and approved APTIVUS within a six month time frame.
Translation:
After years of development and clinical trials, a drug manufacturer has received accelerated approval for its new protease inhibitor Aptivus (tipranavir). The new drug is targeted to those HIV infected people who have been on medications in the past and have developed resistance to currently available therapies.
What is the dosage of Aptivus?
Aptivus is supplied in 250mg soft gel capsules. Two of capsules will be taken along with two 100mg capsules of Norvir twice daily. Aptivus must be taken or boosted with the 200mg of Norvir in order for it to be effective. Aptivus is also to be taken with food!
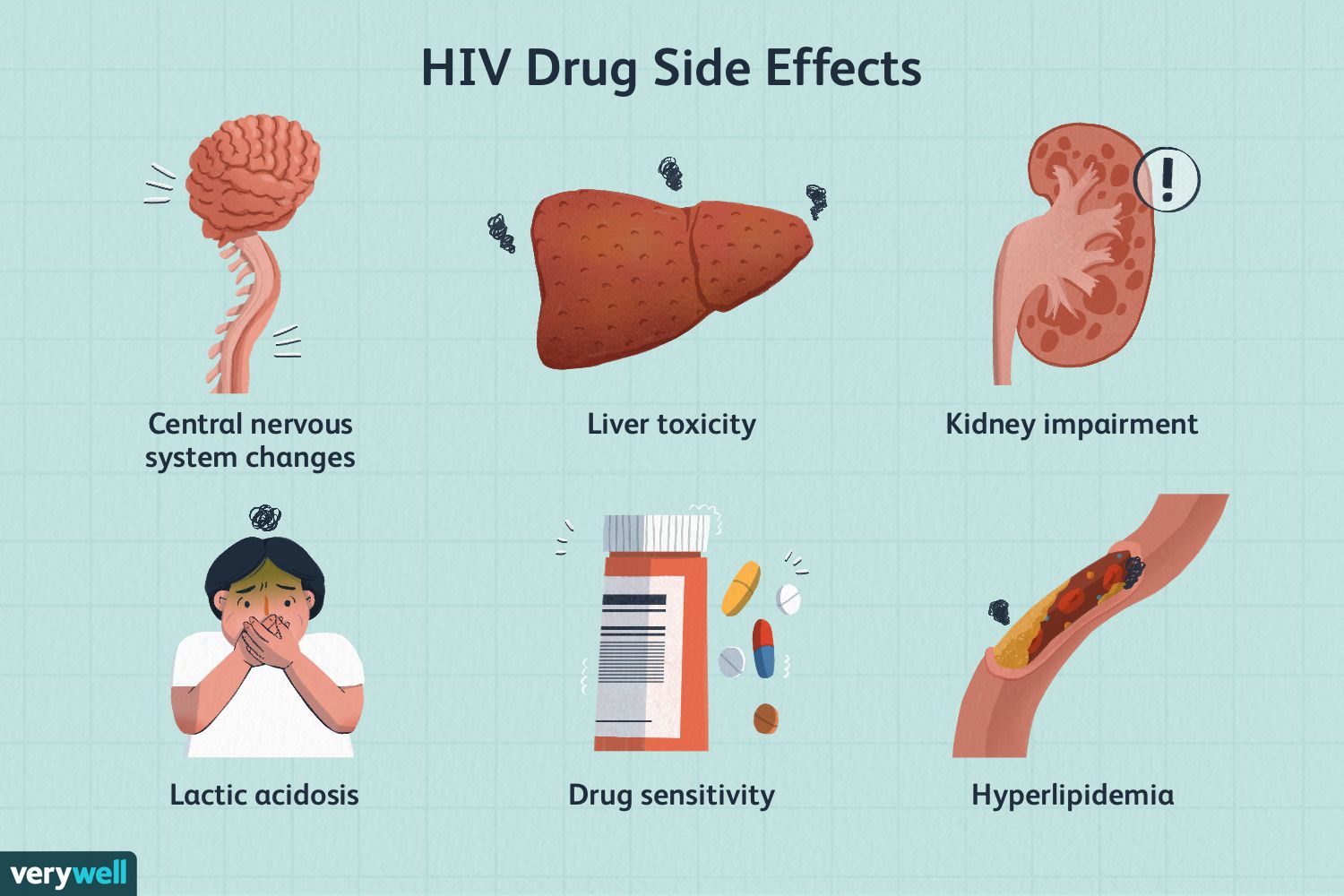
Side Effects Include:
- rash
- diarrhea
- nausea
- vomiting
- headache
- elevated liver enzymes and liver toxicity
Precautions and or Drug Interactions:
Like many medications, there are some precautions prescribers and users must be aware of before starting Aptivus. They include:
- Liver toxicity: patients with known liver disease or insufficiency, including those with hepatitis a, b, or c should use Aptivus with extreme caution due to the incidence of liver toxicity caused by Aptivus.
- Sulfa Allergy: patients with sulfa or Bactrim allergies should use Apivus with caution due to sulfa components in the drug that may trigger symptoms of sulfa allergy.
- Drug interactions: many drugs such as St. John’s Wort, anti-arrhythmic drugs, cholesterol lowering drugs, migraine medicines, and herbal remedies can have serious interaction with Aptivus. The prescribing physician should be made aware of all medications and herbal products being taken prior to prescribing Aptivus.
- Birth control pills and patches: additional methods of birth control should be used when taking Aptivus due to decreased affectiveness of birth control pills and patches caused by this drug.
- Aptivus (tipranavir) should be available in pharmacies within a few weeks. Please note that until this new drug appears in drug formularies, insurance coverage may be delayed. This drug should be in formularies within a month or two.
- Cases of AIDS in the USA
- Food Safety for People Living with AIDS
- HIV Rapid Test
- HIV Home Test
- HIV and Gay Men
- What Every Teen Needs To Know About HIV And AIDS.
![]()
For more information:
CDC National AIDS Hotline
1-800-342-AIDS
Spanish: 1-800-344-SIDA
Deaf: 1-800-243-7889
CDC National Prevention Information Network:
P.O. Box 6003
Rockville, Maryland 20849-6003
1-800-458-5231
FDA – www.fda.gov